“Case in which a judgment about the interpretation of “can be carried out” concerning the Enablement Requirement was presented”
Territory:Japan
Practices:Patents
Category:Cases
Japan Patent Information Committee
SHIBATA, Tomohiko
-Heisei 26 (2014), (Gyo-Ke) No. 10238. A case seeking revocation of an appeal decision-
Against the subject case, the Japan Patent Office issued a decision at appeal rejecting the invention titled “Activated Foam”; and as a result of a law suit filed with the Intellectual Property High Court seeking revocation of the appeal decision, the appeal decision was revoked. That is, the grounds for revocation that had been asserted by the plaintiffs were accepted. The IP High Court rendered a judgment that held as follows: enablement of the subject invention under Article 36, Par. 4, item 1 of the patent law should be construed to mean that a product can be made and used, and if any person skilled in the art can make the product and use the same in an embodiment that at least has a certain technical significance, even without any specific relevant description appearing in the specification but based on the disclosures of the specification, etc. and on common technical knowledge as of the filing of the subject application, it can be said that the above-noted Enablement Requirement is satisfied (the judgment rendered on August 5, 2015).
While the judgment made by a large collegial body of the IP High Court and identified as Heisei 17 (2005), (Gyo-Ke) No. 10042 presented criteria for issuing a judgment with respect to the Support Requirement in patent examination and is now widely known, the subject case presents criteria for judgment that relates to the Enablement Requirement. Thus, the judgment rendered should be referred to.
(1) Subject Application
The subject application is Japanese Patent Application No. 2006-536494 (Title of Invention: Activated Foam; Filing Date: May 16, 2005 (Priority Date: April 29, (2005)), and Claim 1 pending at the time the appeal decision of rejection was rendered to read as follows:
[Claim 1]
An activated foam that is made of a natural or synthetic rubber or a synthetic resin and which comprises a foamed sheet having a closed-cell structure, characterized in that said foamed sheet contains a zirconium compound and/or a germanium compound and is used so as to directly or indirectly contact with a human body when a pharmaceutical agent is administered.
(2) Appeal Decision by the Japan Patent Office
The appeal decision concludes that the specification of the subject application does not satisfy the requirement stipulated in Article 36, Par. 4, item 1 of the patent law for thereason that it does not provide any description that enables those skilled in the art to understand and recognize the combined effect of using a pharmaceutical agent and the activated foam.
(3) Judgment by the IP High Court
The IP High Court made a judgment to revoke the appeal decision, finding that it erred in judging that the specification of the subject application does not satisfy the requirement of Article 36, Par. 4, item 1 of the patent law for the reason that it does not provide any description that enables those skilled in the art to understand and recognize the combined effect of using a pharmaceutical agent and the activated foam of the invention of the subject application.
(3-1) Contents of Enablement Requirement
The IP High Court has presented the following criteria for judgment:
Working of an invention of a product means acts of manufacturing, using, etc. of the product (Article 2, Par. 3, item 1 of the patent law), so enablement of the subject invention under Article 36, Par. 4, item 1 of the same law should be construed to mean that the product can be made and used, and in the case of an invention of a product, the specification is required to provide a specific description about a method for manufacturing the product and a method of using the same; however, if any person skilled in the art can make said product and use the same even without such description but based on the disclosures of the specification, etc. and the common technical knowledge at the time of filing of the application, it can be said that the above-noted Enablement Requirement is satisfied.
Furthermore, in order to be able to say that a product “can be used” as mentioned above, it should be required that a product according to a patented invention can be used in an embodiment that at least has a certain technical significance, as exemplified by a case where it can be used in an embodiment in which the action/effects, etc. that are intended by the invention are exerted.
(3-2) Description in the specification of the subject application
The problems to be solved by the invention of the subject application and the effects of that invention are as follows:
“Problems to be solved by the Invention
…
[0007] …an object of the present invention is to provide an activated foam having no adverse effect and capable of facilitating blood circulation and promoting the improvement of physical condition and the cure of diseases such as cancer.
[0008] As a result of earnest studies for solving the above-described problems, the present invention is accomplished. Specifically, the invention relates to an activated foam that is made of a natural or synthetic rubber or a synthetic resin and which comprises a zirconium compound and/or a germanium compound, characterized in that the foam has a closed-cell structure and is used so as to directly or indirectly contact with a human body when a pharmaceutical agent is administered.
[0009] The activated foam of the invention is used by the direct or indirect contact thereof with a human body, which is more effective when further producing friction between the foam and the body. The activated foam of the invention can facilitate blood circulation and promote the improvement of physical condition and the cure of diseases, but a mechanism therefor has not been elucidated.
…
[0011] The activated foam of the invention may be used, in administering a pharmaceutical agent, by directly or indirectly contacting it with a human body to increase the effect of the pharmaceutical agent. In addition, even a pharmaceutical agent which would exert an adverse effect in a large dose, if it is combined with the activated foam, may reduce the adverse effect because its dosage can be decreased.”
“Advantages of the Invention
[0024] According to the invention, in administering a pharmaceutical agent, the activated foam may be used by directly or indirectly contacting it with a human body to increase the effect of the pharmaceutical agent. In addition, even a pharmaceutical agent which would exert an adverse effect in a large dose, if combined with the activated foam, may reduce the adverse effect because its dosage can be decreased.”
Furthermore, Examples provide the following description:
“[0035]
<Test 1>
The activated foam was then used to perform a test for determining effects thereof on body surface contact pressure and blood flow in a human. A method for measuring body surface contact pressure and blood flow will first be described.
[0036]
[Test Subject]
One female aged in her fifties was used as a subject.
[0037]
[Test Method]
The test was carried out using an instrument for measuring blood flow and body surface contact pressure (AMI3037-2, from AMI Technology). A body surface contact pressure/blood flow sensor was attached to the upper part of the thigh for measurement in the environment of a room temperature of 23 °C and a humidity of 55% RH under the following two conditions. In condition 1, the activated foam was spread on a chair, on which the subject then sat in resting state for 30 minutes, followed by measuring blood flow, blood volume, blood velocity, and body surface contact pressure for 10 minutes. In condition 2, the subject sat, for control, in resting state on a chair on which the activated foam was not spread for 30 minutes, followed by measuring blood flow and the like for 10 minutes. The results are shown in Table 2…. Table 2 shows the mean values of measurements of blood flow and the like for 10 minutes.… In this respect, “blood flow” refers to blood flow per 100 g of human body tissue per minute, and is determined from the amount of light reflected by red blood cells in capillary vessels. “Blood volume” refers to blood volume per cross-sectional area of 100 g of human body tissue, and the product of the blood volume and the blood velocity becomes nearly equal to the blood flow.[0039]
[Table 2]
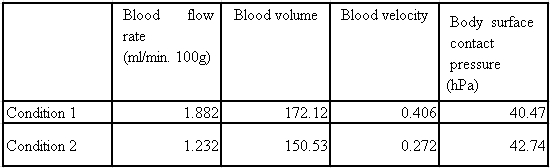
[0040] As shown in Table 2…, the use of the activated foam improves blood circulation and reduces body surface contact pressure.
(3-3) Discussion on whether the activated foam can be used
The IP High Court made the following review of <Test 1> in the specification of the subject application and judged that “depending on the review, there can at least be room to admit that the activated foam of the invention of the subject application ‘can be used’ in such a manner that the blood circulation facilitating effect can be exerted.”
Although this test was conducted in an embodiment in which the activated foam was used “by directly or indirectly contacting it with a human body,” the nature of the activated foam that was used in this test is not described in the specification of the subject application and hence is unclear. Furthermore, the subject was only one female aged in her fifties, and in light of common technical knowledge available to those skilled in the art at the time of filing of the subject application, it is difficult to say that the test results can be evaluated as something objective that is applicable to human bodies in general, and the details of the test conditions are also unclear. Hence, it is not possible to immediately verify whether the effect of the subject activated foam, i.e. “the use of the activated foam improves blood circulation and reduces body surface contact pressure,” which is alleged to be demonstrated by the results of measurements of the blood flow rate and body surface contact pressure in this test, and is attributable to the use of said activated foam or to any other factors.
Accordingly, given the results of <Test 1> alone, it is questionable whether conclusion can be made that “the use” of the activated foam “by directly or indirectly contacting it with a human body” has any technical significance; i.e. whether it can be expected to improve blood circulation in a human. It should, however, be noted that depending, for example, on an explanation about the conditions of Test 1, and the presence or absence of other test results and their contents, there remains room to state that sufficient support exists for the above-mentioned technical significance in use of the activated foam of the invention of the subject application.
(3-4) Judgment about the Appeal Decision
The IP High Court held as follows concerning the appeal decision:
Judging from the wording, the phrase “when a pharmaceutical agent is administered” in the claim of the invention of the subject application merely specifies the timing of the use of the activated foam and the claim does not specify the purpose or usage of the activated foam, such as increasing the efficacy of the pharmaceutical agent or promoting the cure of the disease. Therefore, it cannot be said that in determining whether the invention of the subject application satisfies the Enablement Requirement or not, pharmacological actions concerning the combined effect of using the pharmaceutical agent and the activated foam must be supported pursuant to the case of a medicinal use invention.
Hence, even if the specification of the subject application fails to sufficiently disclose the combined effect of using the pharmaceutical agent and the activated foam, if it can be said that there is some other technical significance in using the activated foam “by directly or indirectly contacting it with a human body when a pharmaceutical agent is administered,” it should at least be held that as far as the Enablement requirement is concerned, any person skilled in the art “can use” the activated foam of the invention of the subject application based on the disclosures of the specification of the subject application and the common technical knowledge at the time of filing of the subject application.
(4) Consequence of the subject application
After a judgment to remand, the subject application was re-examined at the Japan Patent Office and amended twice, and then an appeal decision to grant a patent to the subject application was rendered on March 22, 2016 (Patent No. 5932194). Patented Claim 1 now reads as follows:
[Claim 1]
An activated foam that is made of a natural or synthetic rubber or a synthetic resin and which comprises a foamed sheet having a closed-cell structure, said foamed sheet containing a zirconium compound and/or a germanium compound, characterized in that said activated foam is used as placed directly or indirectly under a human body thereby to facilitate blood circulation in a part of the human body with which the activated foam directly or indirectly contacts.
Authors
Patent Division
Japan Patent Information Committee
[Practices]
Patents
Other legal updates authored by Japan Patent Information Committee
Legal updates on Patents
Telephone
+81(3)3270-6641

